Morphpsys, a German biotech company, has moved closer to its goal of getting the first antibody drug out on the market as its licensee corporation, Johnson & Johnson unit Jenssen, reported strong results from a psoriasis drug phase 3 trial, Reuters reports.
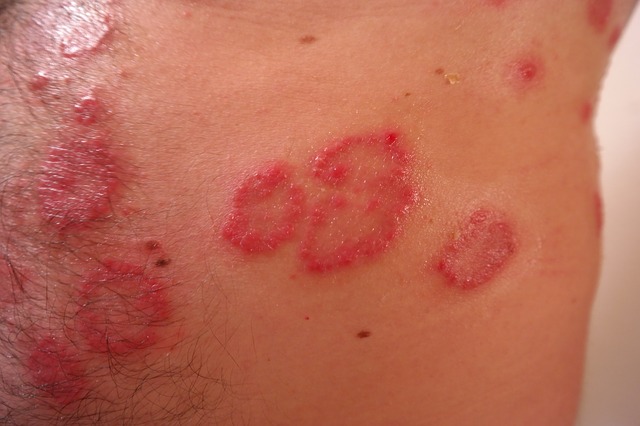
The drug, guselkumab, outperformed a rival company, putting Johnson & Johnson in a position to expand its range of immune-disease treatments. Guselkumab cleared or almost cleared the skin of 81% of patients who underwent 48 weeks of treatment, the company reported. This was a much larger success rate compared to the drug Humira from AbbVie, which recorded just 55%.
Johnson & Johnson’s second-best medication in 2015 sales, Stelara, is already an approved treatment for psoriasis, a condition that causes itchy, scaly skin. Remicade, the healthcare company’s top-selling treatment, is in direct competition with Humira as therapy for a number of conditions, including psoriasis. Cheaper imitations of Remicade called biosimilars threaten to upend sales, prompting Johnson & Johnson to look for a new, more effective drug.
Humira and Remicade both work by halting the activity of a protein called tumor necrosis factor that triggers inflammation. Inflammation is an immune process that overreacts in psoriasis and other similar conditions, such as rheumatoid arthritis and Crohn’s disease – both of which the drugs are able to treat.
Guselkumab, on the other hand, specifically targets a protein called interleukin-23, which is more closely involved in the skin’s immune response. Andrew Blauvelt, president of the Oregon Medical Research Center and lead scientist on the Johnson & Johnson study, explained to Bloomberg,
We’re targeting the disease better.
He says guselkumab has better efficacy compared to Humira.
Among the study’s 837 participants, 4.9% of those on guselkumab experienced serious side effects while 4.5% experienced the same on Humira, after 48 weeks. But 85% of patients on guselkumab had clear or nearly clear skin after 16 weeks, compared with 6.9% of patients on placebo. For those on Humira, 73% reported nearly clear skin compared to 2.9% on placebo.
Two patients – one in each group – suffered a heart attack during the study, and two using guselkumab developed prostate cancer and breast cancer, compared with none among those on Humira. Blauvelt said the cancers, which are relatively common, were not likely to have been drug-related and that long-term safety studies are necessary.
The study was presented at the European Academy of Dermatology and Venereology Congress in Vienna.