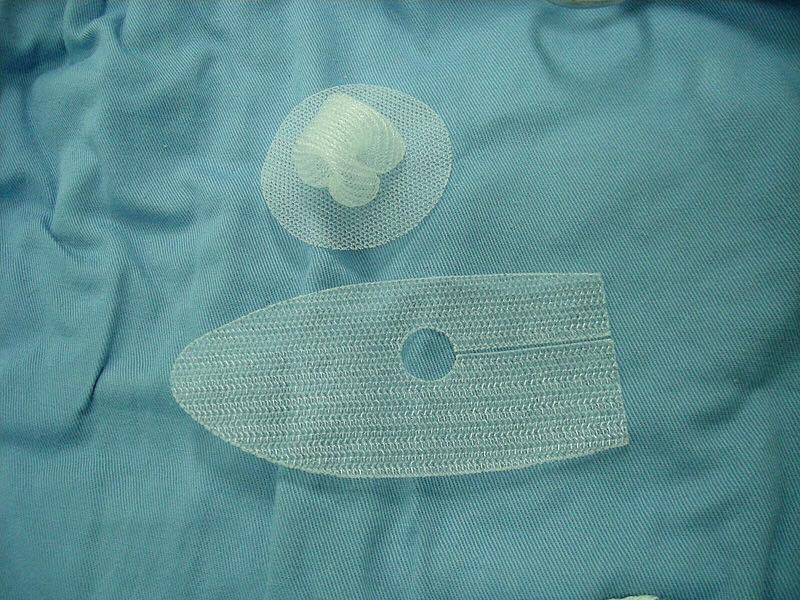
Over 700 women in Australia are bringing a class-action suit against pharmaceutical retailer Johnson & Johnson, claiming that the company’s vaginal mesh implants gave them severe pain, ruined their bodies, and in some cases, destroyed their lives.
In countries such as the United States, Canada and the United Kingdom, thousands of other women have filed lawsuits against the company, for the same reason. The pelvic mesh devices are used to treat urinary incontinence and supposedly repair pelvic organ prolapse, a condition that occurs when childbirth shifts organs out of place, US News reports.
Patients who have sued the manufacturers reported that the mesh caused them chronic pain, infections, incontinence and loss of sexual abilities. In 2014, Endo International, based in Ireland, settled over 20,000 personal injury lawsuits amounting to around $830 million to settle similar claims made against their mesh devices.
The Australian trial, which began Tuesday, is expected to last six months. According to the suits, Johnson & Johnson failed to properly warn doctors and patients about the risks the devices might cause. In addition, the lawsuits argue that the products were not suitable for the purposes they were designed to fit, and testing was inadequate before the mesh implants reached the market.
Jan Saddler of Shine Lawyers, the firm representing the women, said that the primary problem was that the devices erode into the surrounding tissue and organs, causing a chronic inflammation. Almost all of the women involved in the suit complained of perpetual pain, and are said to have suffered relationship issues due to their lack of sexual function.
Saddler said,
Many women are no longer able to have any sort of sexual relationship, or if they are able to have a sexual relationship, there is a lot of pain associated with that.
She added, “Women have been also unable to really enjoy proper fulfilling relationships not only with their partners, but with their children, with their friends…Women have found it very difficult to work in the way they used to work. So it’s had really debilitating impacts.”
The manufacturer has sold over 100,000 mesh products in Australia. The lawsuit points to nine separate devices, five of which Johnson & Johnson have removed from the market. None of the products have been recalled by Australian regulators.
In the USA, the Food and Drug Administration reclassified all pelvic mesh implants as “high risk” and not “moderate,” meaning they need to undergo extra regulatory scrutiny. However, there has been no recall, as well.